S D P Block Periodic Table
Since s subshell can accommodate only two electrons these elements usually have one group i or two group ii electrons in the outermost shell.
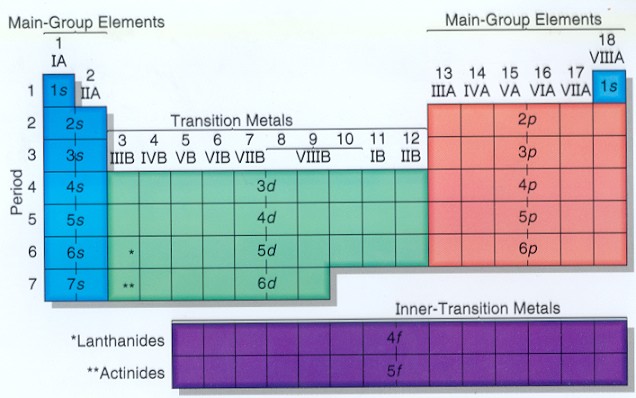
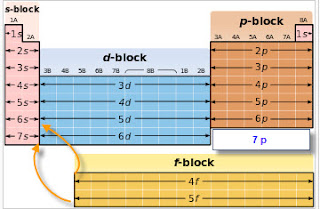
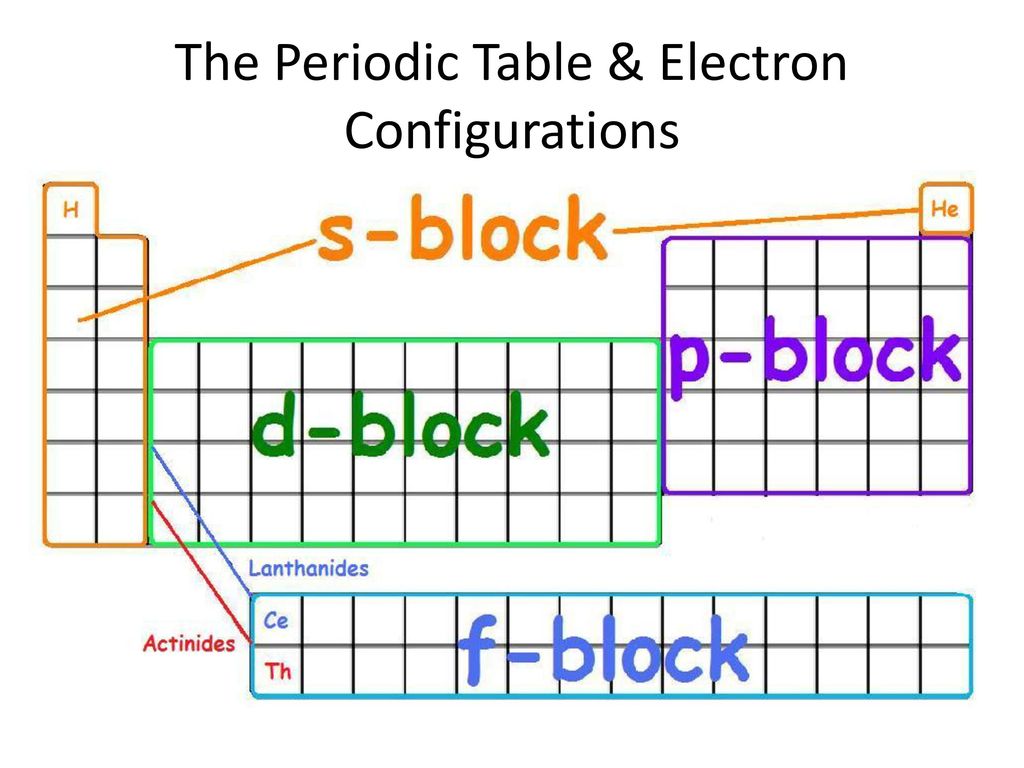
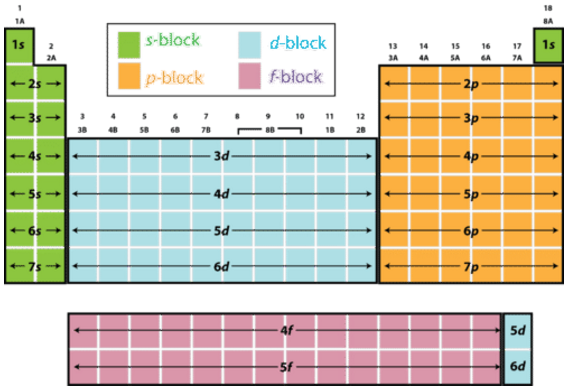
S d p block periodic table. Group i elements are known as alkali metals. The periodic table shows us the sequential filling of the electrons the energy of the orbitals determines the sequence of filling lower energy orbitals are always preferred over high energy ones the table is thus divided into 4 blocks namely s p d f blocks depending on the occupation of the respective orbitals by the valence electrons of an element. S block elements are the chemical elements in group i and group ii in the periodic table.
For example the s block has the numbers on my periodic table la and iia the p block has numbers from 3b to 7b d block from 3a through 8a ending with l b and ii b f block on my periodic table the whole f block falls under the 0. The elements in group i and ii are shown above in the table. The periodic table of elements can be organized by blocks s p d f g.
Recent posts wooden sofa set indian sofa cama 2 plazas precios. The d block is in the middle of the periodic table and encompasses elements from groups 3 to 12. S block elements general electronic configuration is ns 1 2 this block is situated at the extreme left of the periodic table and contains elements of group 1 and 2.
In biomedical sciences and is a science. Most or all of these elements are also known as transition metals because they occupy a transitional zone in properties between the strongly electropositive metals of groups 1 and 2 and the weakly electropositive metals of groups 13 to 16. It starts in the 4th period.
Learn what element blocks are and their properties and characteristics. The division of elements into blocks is primarily based upon their electronic configuration as shown in fig. Group 2 elements occur directly to the right of group 1 elements.
The highest energy level valence shell contains only 1 electron in an s subshell. Group 1 elements occur at the beginning of a new row period of the periodic table. Division of periodic table into s p d and f blocks on the basis of electronic configurations the long form of periodic table can be divided into four main blocks.
Helmenstine holds a ph d.
/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)

/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)