S D P F Orbitals
The orbitals p d and f have separate sub levels and will thus accommodate more electrons.
S d p f orbitals. F orbitals can have 7 orientations in space. Group 1 elements occur at the beginning of a new row period of the periodic table. All we have to do is remember the shapes that.
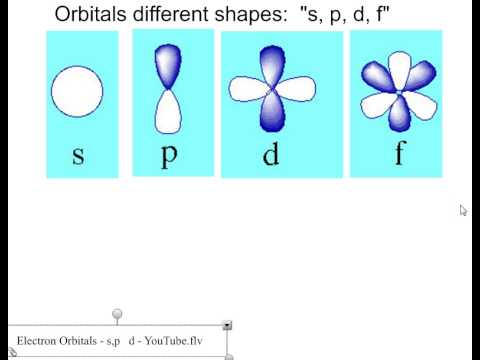
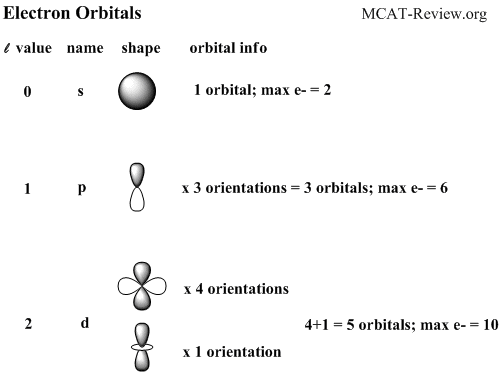
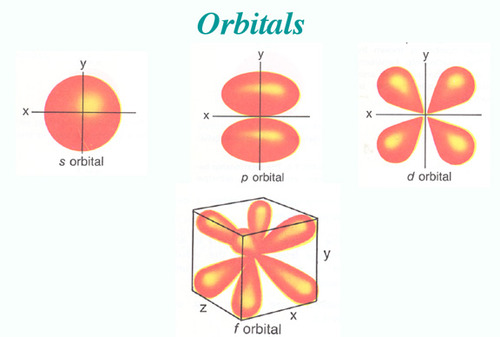
There are four types of orbitals. D orbitals can have 5 orientations in space. The orbital names s p d and f describe electron configuration.
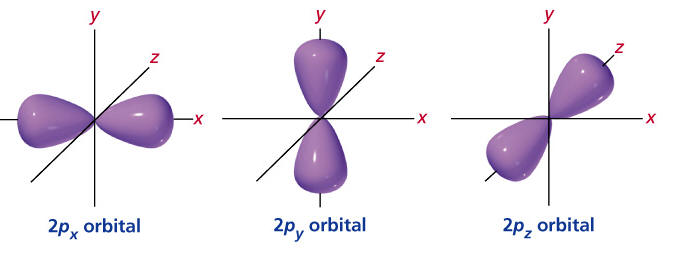
For the s orbital you can have 2 electrons. S orbitals only have 1 orientation in space. The orbital letters are associated with the angular momentum.
The four different orbital forms s p d and f have different sizes and one orbital will accommodate up to two electrons at most. Thus s orbital corresponds to spherical shape with the atomic nucleus at its centre. Group 2 elements occur directly to the right of group 1 elements.
For the d 10 electrons. The letter refers to the shape of the orbital. The labels s p d and f blocks of the periodic table refer to the subshell that is being filled with electrons.
These line groups are called sharp principal diffuse and fundamental. As shown each element s electron configuration is unique to its position on the periodic table. For the p orbital you can have 6 electrons.
The letters s p d and f were assigned for historical reasons that need not concern us. For s orbital azimuthal quantum number 0 and the magnetic quantum number m 0 hence s orbitals have unique orientation in space. How do orbitals work.
The letters go in the order s p d f g h i j etc. The orbital names s p d and f stand for names given to groups of lines originally noted in the spectra of the alkali metals.